Directly Labeled
- Determine concentration of Antibody (Ab) and centrifuge 10 min at 15,000 X g, 4° C to remove aggregates. Discard pellet.
- Start with Ab stock at 300 µg/ml in Phosphate Buffered Saline (PBS). Serial dilute six tubes:
10 µl Ab + 20 µl PBS (or 20 µl of previous dilution) = 30 µl
- Resuspend cells in 200 µg/ml normal IgG of detecting species at 5 - 10 X 106 cells/ml.
- Aliquot 50 µl of cells in (Falcon #2054) 12 X 75 mm tubes on ice.
- Add 10 µl of each Ab dilution. Also prepare a tube with cells alone (autofluorescence control) and one with the detecting species IgG negative control.
- Gently mix and incubate 15 - 45 min on ice IN THE DARK.
- Add 2 ml cold Washing Buffer (e.g. PBS + 2% Bovine Serum Albumin), gently mix and centrifuge 5 min at 200 X g, 4° C.
- Aspirate and discard supernatant. Gently vortex to break up cell pellet and repeat Step 7.
- Resuspend pellet in 100 µl protein-free, cold PBS and mix well.
- Add 300 µl cold PBS + 2% paraformaldehyde. Store in refrigerator, light protected for up to 10 days. Can skip fixation and use Propidium Iodide at 0.5 µg/ml in Washing Buffer and run within 4 hours.
Indirectly Labeled
- Prepare cells in 200 µg/ml normal IgG of the second antibody species (e.g. goat) at 5 - 10 X 106 cells/ml.
- Start with Ab stock at 300 µg/ml in PBS. Serial dilute six tubes:
10 µl Ab + 20 µl PBS (or 20 µl of previous dilution) = 30 µl
- Aliquot 50 µl of cells in (Falcon #2054) 12 X 75 mm tubes on ice.
- Add 10 µl of each Ab dilution. Also prepare a tube with cells alone (autofluorescence control) and isotype negative control.
- Gently mix incubate 15 - 45 min on ice.
- Add 2 ml cold Washing Buffer (e.g. PBS + 2% BSA), gently mix and centrifuge 5 min at 200 X g, 4° C.
- Aspirate and discard supernatant. Gently vortex to break up cell pellet and repeat Step 6.
- Resuspend cells in 100 µl of appropriately diluted fluorochrome-conjugated second antibody (e.g. goat anti-mouse FITC).
- Incubate 15 - 45 min on ice IN THE DARK.
- Wash twice as above (Steps 6 & 7).
- Resuspend pellet in 100 µl protein-free, cold PBS and mix well.
- Add 300 µl cold PBS + 2% paraformaldehyde. Store in refrigerator, light protected for up to 10 days. Can skip fixation and use Propidium Iodide at 0.5 µg/ml in Washing Buffer and run within 4 hours.
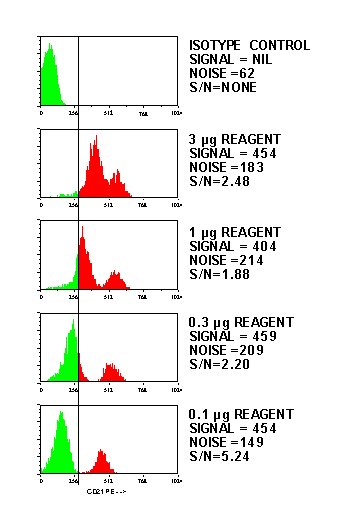
Here's an example of a titration showing the expected decrease in fluorescence with a corresponding increase in the signal to noise ratio:
Reference
Current Protocols in Cytometry, J Wiley & Sons, N.Y., eds. Robinson, J.P. et al, 4.1-4.4, 1997.