2019 MOMI Seeds
Page Content
The second request for MOMI Seeds pilot grant proposals closed on October 15, 2018, and we received a total of 16 applications. Six of them are revised applications that did not get funded in the first round; the other ten applications are new project proposals. Thus, together with the 27 applications submitted in the first round, we received a total of 37 unique project proposals from UC San Diego investigators over the past two rounds combined, highlighting the immense interest in and potential for human milk and lactation research at UC San Diego and emphasizing the high demand for additional funding in this critical line of research.
16
| Number of Applications Submitted
|
$250, 000
| Research funds awarded
|
5
| Number of awards granted
|
2019 Awardees

| Robert H Tukey, PhDProfessor, Department of Pharmacology
"Human breast milk induces neonatal hyperbilirubinemia" Newborn children, both preterm and term, are susceptible to the development of neonatal hyperbilirubinemia which in certain situations can lead to acute and chronic forms of encephalitis. The development of hyperbilirubinemia, especially in preterm children, can be directly linked to breast milk. Our laboratory has developed “Humanized” animal models that express the gene and protein that metabolizes serum bilirubin, allowing us to directly investigate the underlying components of breast milk that induces neonatal hyperbilirubinemia. The identification of the components of breast milk and the mechanisms that lead to hyperbilirubinemia will provide valuable insight into the future development of therapeutic tools that can be leveraged to limit the toxicity associated with breast-milk induced neonatal jaundice.
Dr. Tukey is a Professor of Pharmacology and Director of the UC San Diego Superfund Research Center. His background stems from expertise in several areas of Molecular Pharmacology with an emphasis in mouse genetics and the use of mice to examine the molecular events underlying the tissue specific and inducible expression of human UDP-glucuronosyltransferase (UGT) genes. The human UGT1A1 gene is developmentally regulated in mice and its expression leading to the metabolism of serum bilirubin will be employed to identify the key components of human breast milk that lead to the induction of neonatal hyperbilirubinemia.
|

| Amy Non, MDAssociate Professor, Department of Anthropology
"Epigenetics in longitudinal breast milk, maternal mental health, and child growth" Breastfeeding is an important mechanism to transmit nutrition and immunity to offspring, but can also transmit maternal signals of psychosocial stress across generations. These signals can be transmitted in the form of stress hormones, such as cortisol, or via changes in activity of small nuclear molecules, such as microRNAs (miRNAs). These molecules are highly abundant in breast milk, and can potentially alter developmental programming of infant metabolism and growth. We propose to study associations between maternal perceived stress, depression, and anxiety on milk cortisol, miRNA expression levels, and infant growth across two post-natal time points. We hypothesize that mothers with higher stress, depression, or anxiety will show differential expression of miRNAs, dysregulated cortisol, and reduced infant growth across the postnatal period.
Dr. Non is a molecular anthropologist with an interest in genetic and sociocultural contributors to racial and social inequalities in health. Her research investigates how social experiences, such as discrimination, poverty, and other stressors, can become biologically embedded early in life to affect health throughout the life course. Her current focus is on epigenetic mechanisms that can link early adverse environmental exposures with altered gene expression, potentially resulting in long-term health consequences.
|

| Meghan O. Altman, PhDAssistant Project Scientist (PI: Pascal Gagneux, PhD), Department of Pathology
"Do breast milk components protect against current influenza strains?" In addition to providing optimum nutrition, breast milk protects infants against infection and the severest effects of many viruses, including influenza. Milk sugars play a large role in this protection by acting as decoys for the specific sugars viruses themselves use to begin infection. Harnessing the unique immune system of an aquatic animal called the lamprey, we were surprised to discover the viral sugars on influenza’s surface are also found in large quantities in human milk. This project will determine if these milk sugars act as decoys and alter influenza infection. If successful, our project will reveal a new way breast milk impacts influenza infection, and will develop an experimental foundation to identify additional milk sugars with potential for altering interactions with other pathogenic or commensal microbes important for infant health.
Dr. Altman conducted her PhD research at the University of Florida, received her postdoctoral training at the National Institutes of Health, and is now working as a Project Scientist under Dr. Pascal Gagneux in the Pathology Department at UC San Diego. She is an expert in the specialized sugar molecules found on the surface of the influenza virus. Her research attempts to exploit biases in the evolution of these sugars to forecast upcoming changes in influenza. These forecasts could improve hospitals’ annual flu season preparation and help select better vaccine strains. While laying the foundations for this research, she breastfed her own children, and is grateful for the medical, community, and workplace support that made this possible.
|

| Bernd Schnabl, MDProfessor, Department of Medicine
"The role of fucose metabolism for obesity and non-alcoholic fatty liver disease" Obesity and non-alcoholic fatty liver disease (NAFLD) are common diseases with increasing prevalence due to over nutrition and sedentary life style. Until recently, mechanistic studies have largely focused on patient physiology, but new work has demonstrated dramatic changes in the microbiota of patients with obesity and NAFLD. The aim of this proposal is to learn from human milk and determine the effect of fucose metabolism on obesity and NAFLD with the goal of identifying new targets for the treatment of these debilitating diseases.
Dr. Schnabl is a trained gastroenterologist and physician-scientist. He joined the Division of Gastroenterology at UC San Diego in 2008 and he is currently Professor of Medicine in Residence. His lab is particularly interested in the contribution of the gut microbiome to the onset and progression of metabolic and liver diseases. He is using system biology approaches to better understand the interaction between the intestinal microbiome and the liver.
|

| Kathryn Patras, PhD
Postdoctoral Fellow (PI: Victor Nizet, MD), Department of Pediatrics
"Investigating the use of breast milk components to control Group B Streptococcus vaginal colonization Group B Streptococcus (GBS) colonizes the vaginal epithelium of a significant percentage of healthy women, but during pregnancy, GBS creates risk of serious disease including preterm birth or neonatal sepsis. Current prevention of GBS disease consists of administering broad-spectrum antibiotics to GBS-positive mothers which produces several unintended consequences including altered neonatal gut bacteria and increased risk for other types of infection. Human breastmilk is a rich source of antimicrobial agents, and previous studies have found that breastmilk inhibits GBS growth. This proposal seeks to examine whether breastmilk components can reduce GBS vaginal colonization using cell culture experiments and an animal model. This project aims to increase our understanding of the biological activities of breastmilk as well as provide a more targeted therapeutic tactic to prevent maternal and neonatal GBS infection.
Dr. Patras is a postdoctoral fellow in Victor Nizet’s lab in the Department of Pediatrics at UC San Diego. In 2008, Katy received a B.Sc. in Animal Science at the University of Nebraska-Lincoln, and completed her Ph.D. in Biology at San Diego State University in 2015. Her graduate work focused on the bacterial and host factors controlling bacterial vaginal colonization, and in postdoctoral studies, she is expanding her focus to include influences of host immunity and metabolism on bacterial urinary tract infections. The overall goal of her research is to improve maternal and neonatal health outcomes.
|
2019 Program Summary
UC San Diego faculty members were eligible to apply. UC San Diego Postdoctoral Fellows, Medical Fellows, and Residents (Trainees) were also encouraged to apply, but needed to be sponsored by a UC San Diego faculty member.
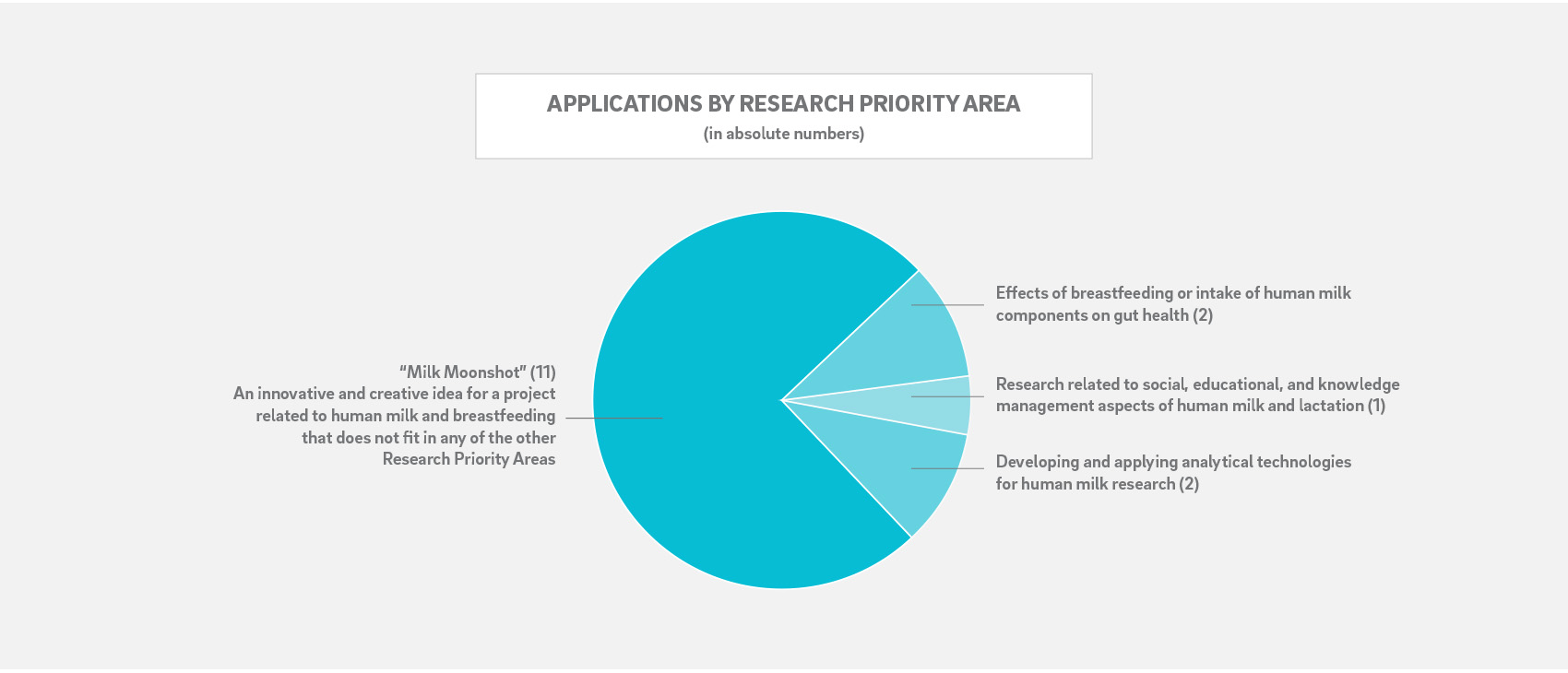
Specific Research Priority Areas (RPAs) are assigned for each application cycle based on the short- and long-term strategic goals of the center. The 2019 cycle invited submissions and received applications with relevance to the following RPAs:
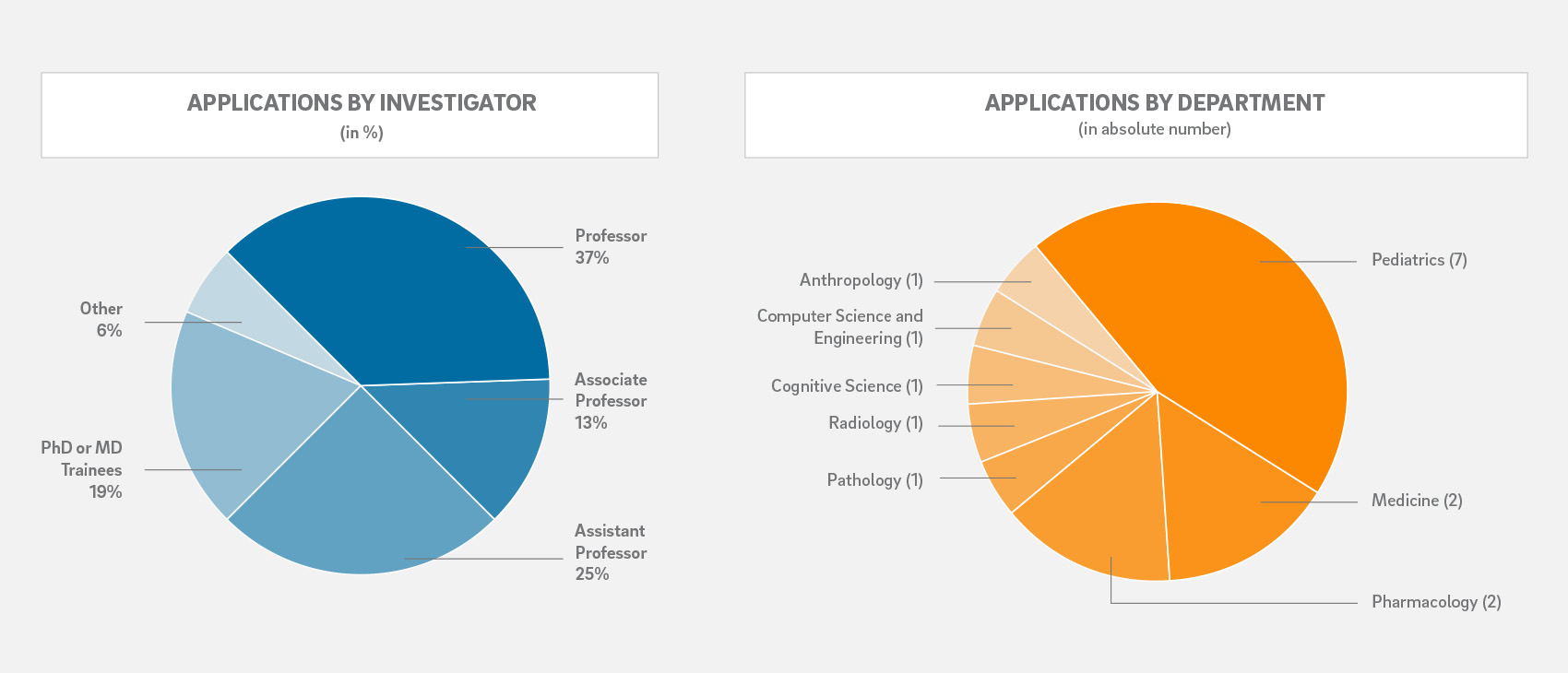
Each application was assigned to two out of eleven independent peer-reviewers who scored the applications based on relevance to the respective Research Priority Area, innovation, investigator(s), approach and scientific rigor, feasibility, as well as potential for extramural funding.
Show additional content areas below
ContentA1